Main content
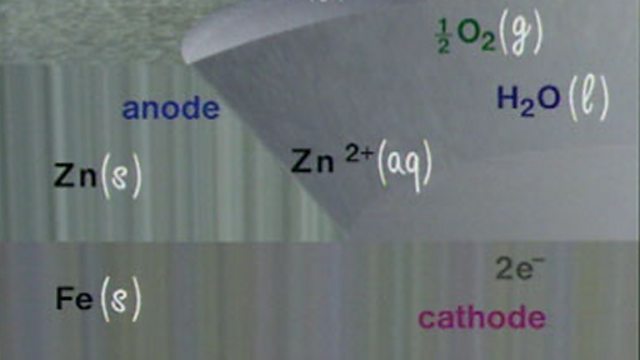
Zinc as sacrificial protection to prevent the corrosion of iron and steel
Sacrificial protection of iron using zinc is explained through a diagram of the electrochemical processes involved. The zinc acts as an anode, reacting with the water and passing electrons to the iron, which does not corrode.
Duration:
This clip is from
More clips from Bitesize: Chemistry
-
![]()
Carbon dioxide in the atmosphere
Duration: 02:08
-
![]()
Recycling plastic
Duration: 01:01
-
![]()
Galvanising iron and steel to prevent corrosion
Duration: 01:10
-
![]()
Alkali metals and their reactions to air and water
Duration: 02:15





